The special chaperonin of the bacteriophage EL
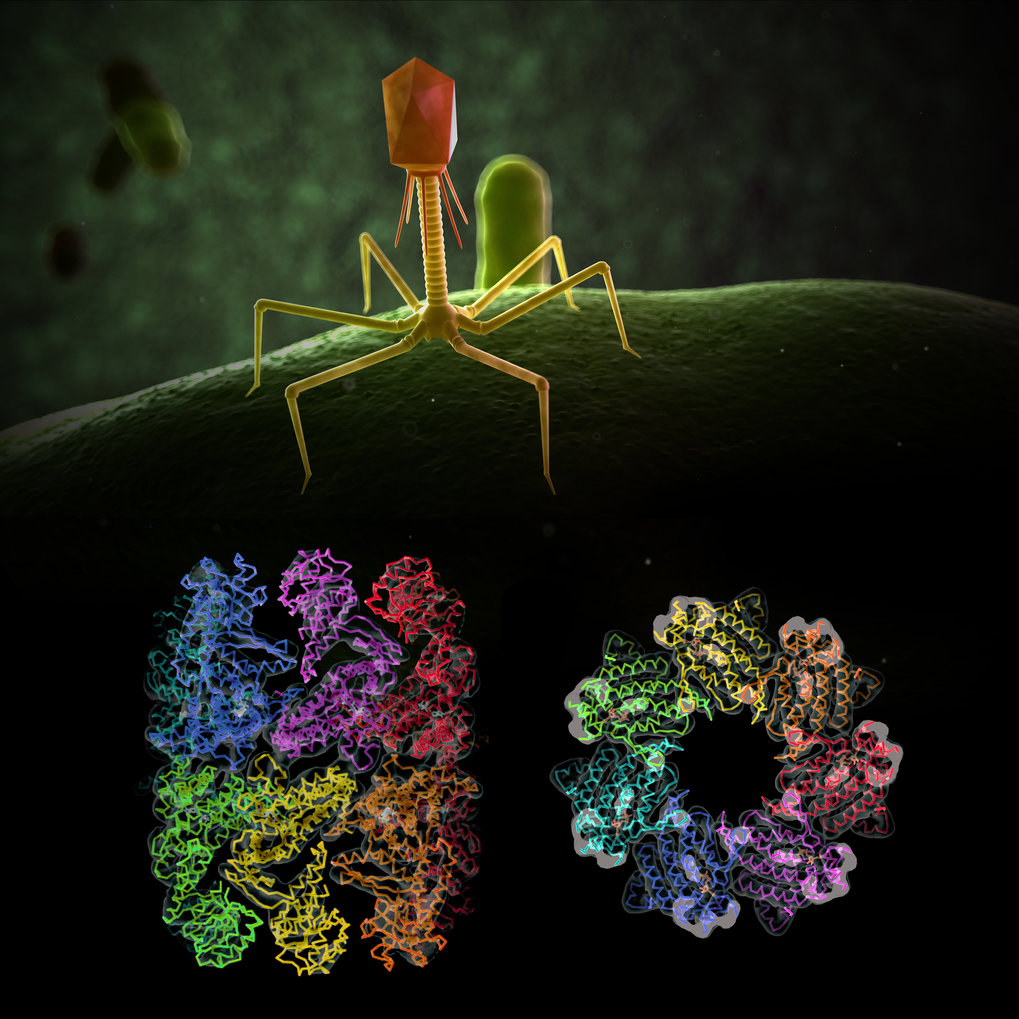
Researchers for protein folding helpers at the Max Planck Institute of Biochemistry have now deciphered the molecular structure of the EL phage chaperonin and discovered special features.
In general, viruses carry only a minimal amount of genetic information. They infect a host cell and use its metabolism and protein production machinery to reproduce. The bacteriophage EL also carries the genetic information of a protein folding helper, a so-called chaperonin. The Bacteriophage EL is a virus that can infect one of the most well-known hospital germs - the bacterium Pseudomonas aeruginosa. Researchers in the team of Manajit Hayer-Hartl and experts for protein folding helpers at the Max Planck Institute of Biochemistry have now deciphered the molecular structure of the EL phage chaperonin and discovered special features. The results of the crystal and cryo-electron microscopy structures show complexes with 7 or 14 subunits that form single and double rings. "In contrast to the known chaperonins, all the structures found were open, which means that they do not form a folding cage as in GroEL/GroES," says Andreas Bracher, first author of the study. "It is possible that this phage chaperonin represents a primitive, evolutionary precursor of today's cellular chaperonins, which works without encapsulation of the substrate proteins". The study was published in the science journal PLOS ONE.
